Antibiotic developer Motif Bio (MTFB:AIM) has been handed a hefty boost after winning regulatory clearance to start Phase III trials for iclaprim.
The treatment is an intravenous formula designed to treat two serious infections, acute bacterial skin and skin structure infections (ABSSSI), and hospital acquired bacterial pneumonia (HABP). Both are bacterias that have built up resistance to most antibiotics, making them potential killers.
Motif listed on AIM last month (2 Apr) to raise the funds needed to help fight multi-drug resistant bacteria. An estimated 50,000 people a year die in Europe and the US from such infections.
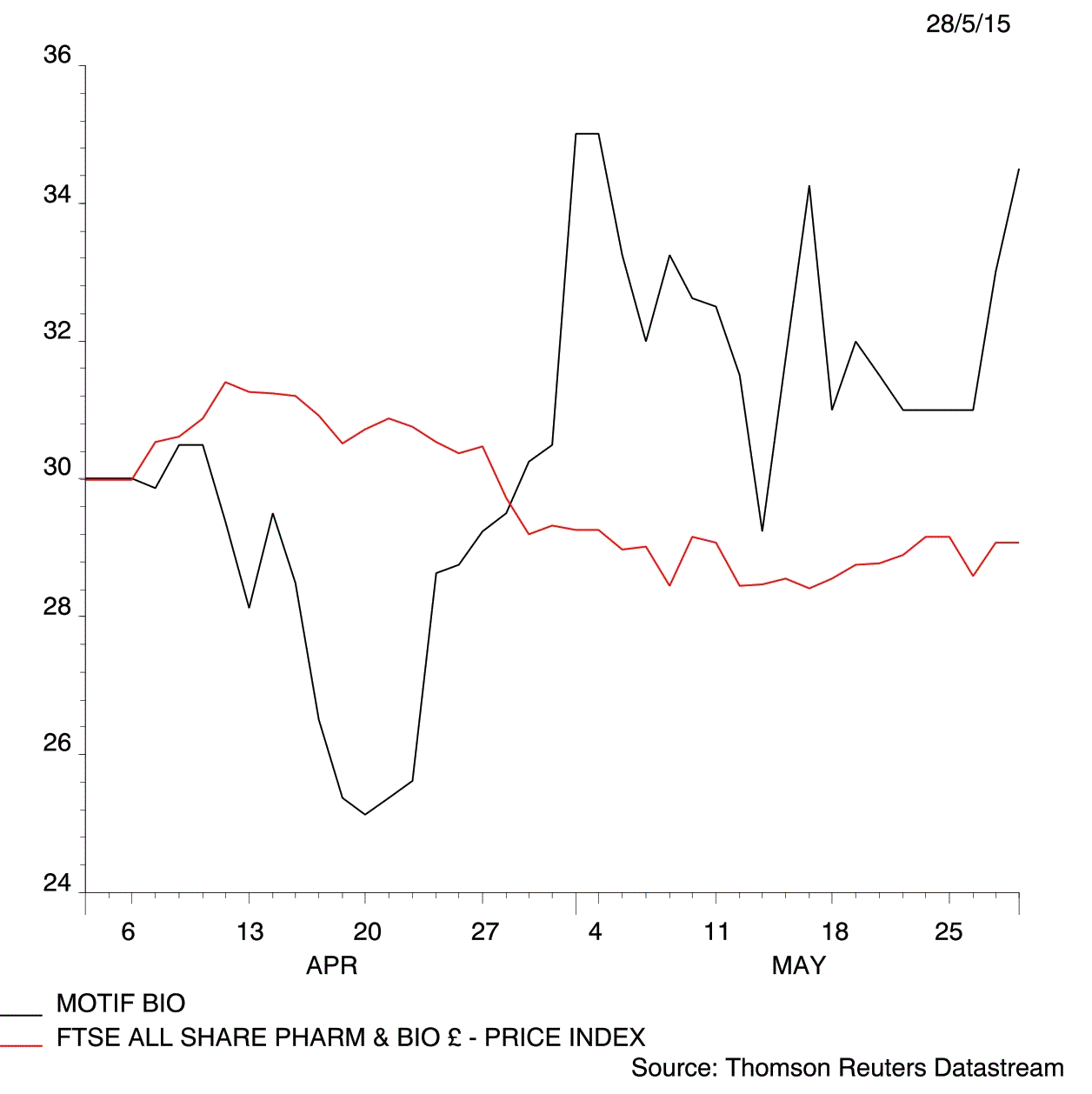
Which makes today's thumbs up from US regulator, the Food & Drug Administration (FDA), all the more timely, inspiring a 4.5% share price rise to 34.5p.
However, the drug trials process is notoriously unpredictable and there can be no guarantee of a positive outcome.
But investors clearly remain hopeful, and if all goes to plan, iclaprim could be being used in hospitals around the globe in a few years time. That could generate sales worth an estimated $1 billion a year, according to some experts.