THIS IS AN ADVERTISING PROMOTION
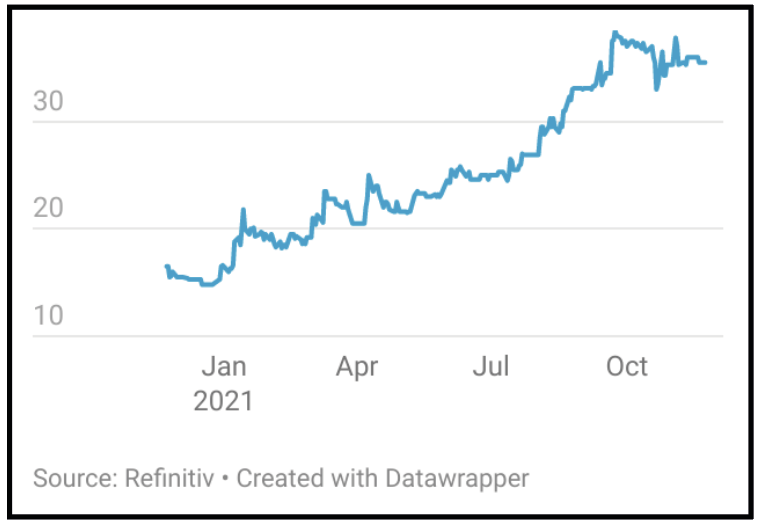
A classic American business investment question that is often asked is where is the sizzle? It is a great question to ask - what really adds value to this? In the case of Allergy Therapeutics (AGY:AIM), the answer is the Pollinex Quattro platform and the Virus Like Particle (VLP) Peanut allergy vaccine candidate. Success or significant progress in either of these pipeline areas is likely to substantially increase the value of the business.
Pollinex Quattro platform
Allergy Therapeutics has just completed a very successful exploratory Phase III trial among patients suffering from grass pollen allergies, to test different placebos, different dosing schedules and scoring methods. This showed an efficacy level of 37%, well above that required by the regulatory authorities. This trial and the learnings from it will enable the group to have a much better chance of succeeding in the pivotal Phase III trial of its investigational, short course injectable immunotherapy, ‘Grass MATA MPL’, which is taking place over twelve months starting October 2022. Results from this study are expected in the autumn of 2023. Grass pollen is one of the most common causes of seasonal allergic rhinitis in the Western world and so success in this trial would open up the possibility, subject to regulatory approval, of a massive opportunity in an undeveloped hay fever market in the US worth potentially between $2-3 billion.
If successful, shares are likely to receive a significant boost from positive results in the trial given the few obstacles that will remain to get the product to market. Following the Grass product, there are also Birch and Ragweed products in development which offer significant opportunities.
Peanut allergy vaccine
If you consider the hay fever market to have big potential, the peanut allergy market could prove to be even bigger, with an estimated US market size of $5 billion. The sort of value this could bring was shown in 2020 when Nestle paid $2.8 billion for US-based Aimmune and its approved peanut allergy product, Palforzia.
Allergy Therapeutics’ next-generation vaccine candidate, VLP Peanut, is due to enter the company’s Phase I ‘PROTECT’ trial in the first half of next year following a very successful laboratory-based ex-vivo study, carried out by Imperial College London, which examined the blood of peanut allergic patients when it was challenged with VLP Peanut or with a peanut extract. The trial showed initial evidence of VLP Peanut’s hypoallergenic potential (i.e. would not cause a patient to have an allergic reaction to it) as well as strong indications of its efficacy potential and ability to induce a protective immune-response. If the PROTECT trial is successful, it would provide further evidence to support the potential of this vaccine candidate to provide a transformational treatment approach for patients suffering from peanut allergy. In contrast to Palforzia, a powder patients need to add to their food or a parent to a child’s food every day to maintain desensitisation, VLP Peanut is being developed as a short-course peanut allergy vaccine. The Allergy Therapeutics product is a short-course series of injections - potentially as few as three. These would be administered in a similar way to the hay fever products, at a clinic and by a specialist doctor. The product has the potential to be disease-modifying, implying that the treatment does not need to be repeated regularly. This would truly be a game-changer in terms of treatment.
Trading business
As well as these two platforms, the group has a successful trading business with revenue of £84.3 million in 2021 and a cash balance at the end of June 2021 of £40.3 million. The group expects to be able to fully fund next year’s pivotal Phase III Grass study, which could prove efficacy to support a US filing with regulators, and the Phase I VLP Peanut trial. There are also four additional, exciting VLP immunotherapy candidates in areas beyond allergy - melanoma, asthma, atopic dermatitis and psoriasis - which use the same technology platform as the VLP Peanut product.
Summary
All in all, this profitable business has plenty of ‘sizzle’ from two biotech type opportunities and a robust trading business.
DISCLAIMER: This article was written by Allergy Therapeutics and published by Shares under a commercial agreement. It is not a recommendation to buy or sell the shares. The article originally appeared in Shares’ Spotlight report on 25 November 2021.