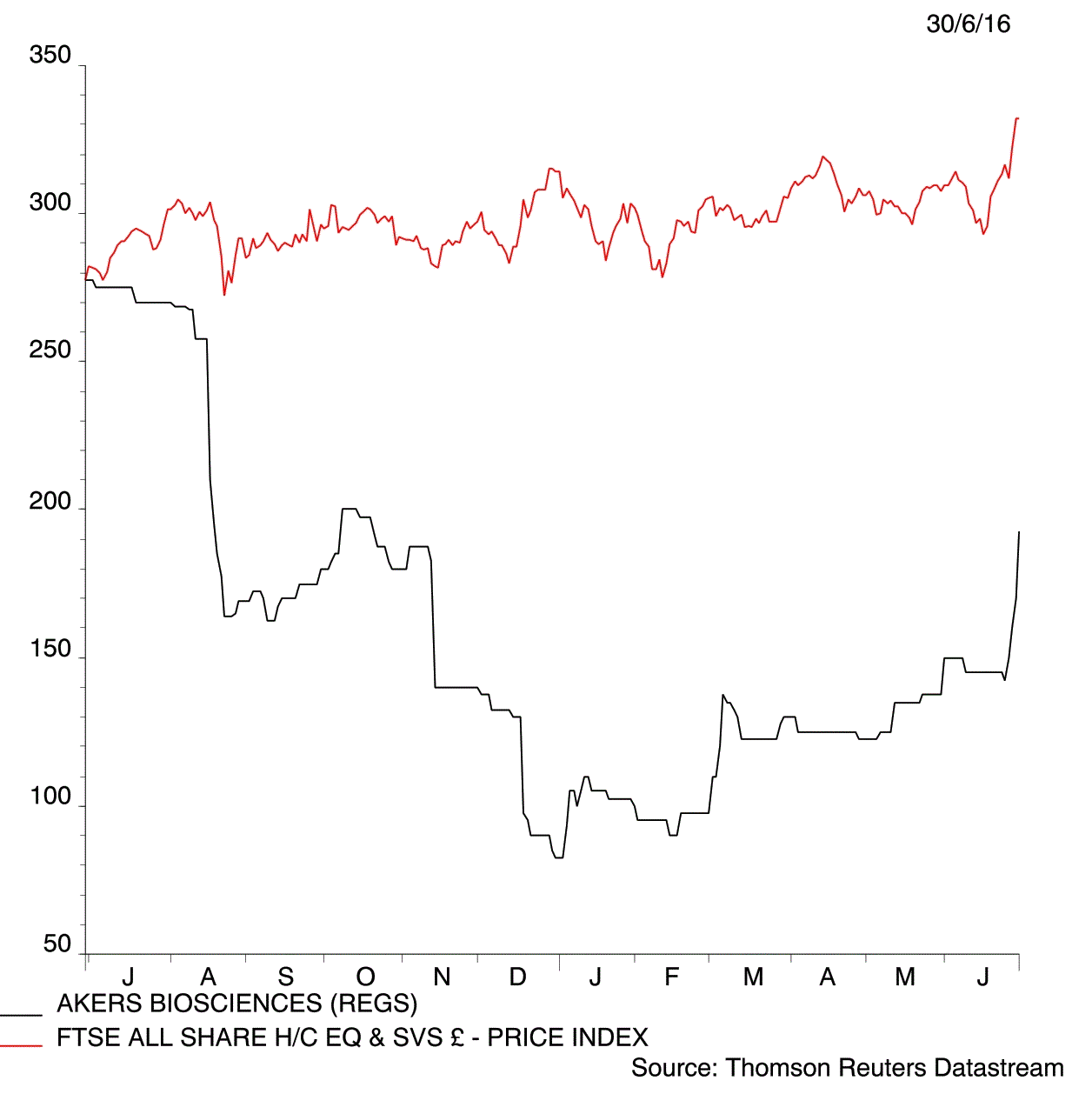
Hopes that Akers Biosciences (AKR:AIM) is on the verge of launching a device that speeds up the diagnosis of Chlamydia has captured the imagination of many investors. Shares in the medical technology developer jump 13.2% to 192p in early trade on Thursday following a 96% success rate at identifying the sexually transmitted disease in clinical trials.
Analysts at FinnCap estimate that US regulator, the Food & Drug Administration (FDA), will make a decision on whether or not the test can be sold across the States during the first half of 2017.
The device is the first of its type capable of diagnosing the condition by using a finger stick blood sample. Traditional methods involve urine samples or genital swabs. Akers’ test takes five minutes to generate a result, compared to 10 days using existing methods in a lab.
This is an example of what technology or pharmaceutical companies should be developing - products that improve on existing methods of treating or diagnosing medical conditions.
Analysts at FinnCap will not consider altering its forecasts until it becomes clear what the initial orders for the PIFA/Chlamydia Rapid Assay will be, but the potential is there.
According to statistics from the US Center for Disease Control & Prevention, there were 1.4 million reported cases of Chlamydia in the US during 2015. That’s around one in every 300 citizens. And that's not including undiagnosed carriers.
It has been a busy few days in terms of news-flow for Akers. On Monday the company closed a deal that will see its BreathScan OxiChek oxidative stress test sold in the US by healthcare services organisation Aero-Med.